Patients who received a Medtronic interventional treatment for hypertension while staying on their blood-pressure pills saw noticeably better results than with a placebo, new results from a small study show.
Medtronic is hotly pursuing a blood-pressure reduction therapy known as "renal denervation," which involves inserting a narrow device into the arteries that lead to the kidneys and then applying radio-frequency energy to zap away nerves in the vessel wall carrying signals that trigger hypertension. High blood pressure is usually treated medically with drugs, but many people don't take their medications as prescribed, past studies have found.
Related
Renal denervation procedure
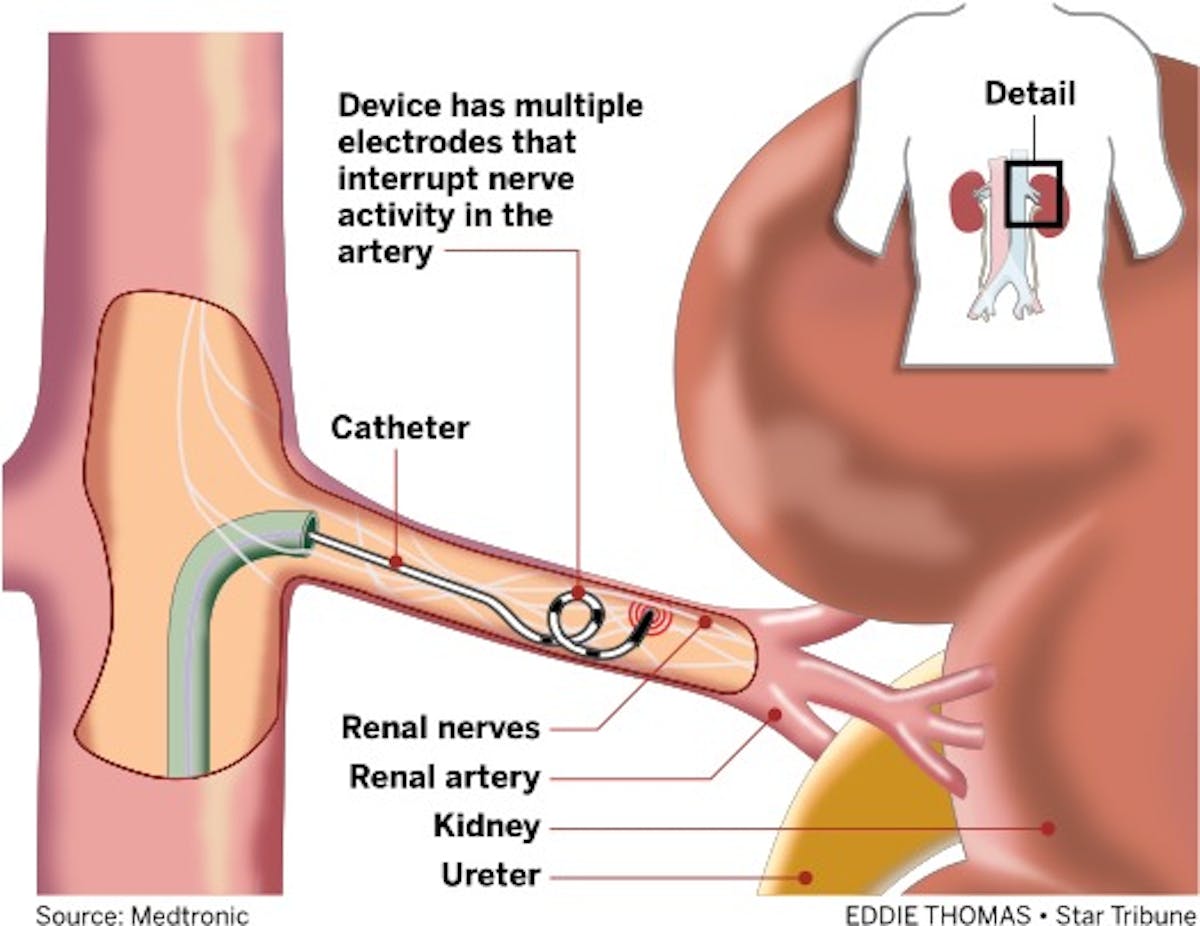
It's difficult to estimate the market value of a medical-device treatment for high blood pressure, since renal denervation is investigational in the United States and researchers are still working to understand which patients respond best. In the past, analysts with Decision Resources Group have estimated that hundreds of thousands of people in the United States could be candidates for the therapy, if approved.
At the EuroPCR meeting in Paris on Wednesday, Medtronic announced positive early results from its proof-of-concept trial comparing renal denervation in patients adhering to their drug prescriptions, to results from a group of patients who also stayed on the drugs but got a "sham" medical procedure that left their renal nerves unaltered.
Based on readings from a continuously worn blood-pressure monitor, drug-taking patients with the sham procedure saw average declines in systolic blood pressure of about 1.6 millimeters of mercury. That compares to declines of 9 millimeters of mercury for patients who got the real treatment and stayed on their meds.
"A 9-millimeter reduction is quite substantial, and quite significant," said Dr. David Kandzari, an Atlanta cardiologist who served as co-principal investigator of the Spyral HTN On-Med study. Kandzari presented the results Wednesday in Paris at the EuroPCR meeting.
The American Heart Association said 10 millimeters of mercury in the systolic pressure can mean the difference between "normal" and "elevated" blood pressure.
The results announced Wednesday summarized six-month outcomes for the first 80 patients randomized in a study of 467 people being treated at hospitals in several countries, including the United States. It's considered an early proof-of-concept trial, and results from a larger study will be necessary to gather enough evidence to get approval from the U.S. Food and Drug Administration. Renal denervation is not approved for use in the U.S. outside of clinical studies.
The "On-Med" study findings were announced about a year after Medtronic unveiled early results of a similar "Off-Med" study, in which patients were taken off their drugs and then randomized to renal denervation or a sham procedure.
That proof-of-concept study found that after three months, patients in the experimental arm with a continuously worn blood-pressure monitor saw an average systolic pressure decrease of 5.5 millimeters of mercury, compared with a 0.5-point reduction in the control group. Based on those results, Medtronic has already launched a larger pivotal trial of renal denervation in patients not taking high blood-pressure drugs.
"We are talking about hundreds of thousands of patients in the U.S., and millions of patients globally, that would qualify under the eligibility criteria in the Off-Med trial," Sean Messenger, a senior manager with Massachusetts-based Decision Resources Group, said in an interview last month. "If the On-Med results are positive, that opens up the pool a lot more."





